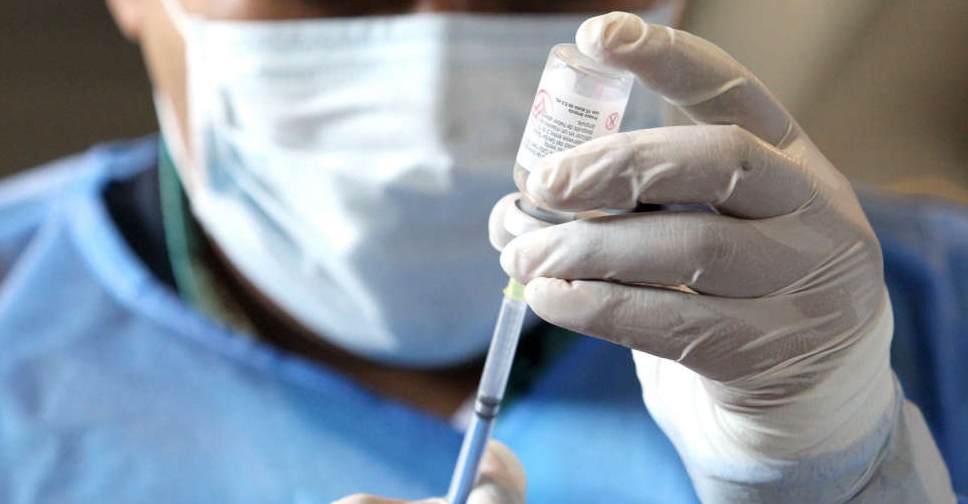
The World Health Organization (WHO) on Thursday issued an emergency use listing for the single-dose COVID-19 vaccine from China-based CanSino Biologics.
The vaccine, Convidecia, is the eleventh shot against the coronavirus to get clearance from the global health agency, whose advisory group recommended its use in people of age 18 years and above.
It was found to have 64 per cent efficacy against symptomatic disease and 92 per cent against severe COVID-19, the agency said.
Other vaccines that have similar clearance from the agency include those made by Pfizer and BioNTech, Moderna, AstraZeneca, Johnson & Johnson and Novavax.




 Syrian government, Kurdish-led SDF agree integration deal
Syrian government, Kurdish-led SDF agree integration deal
 Trump warns Britain on China ties as Starmer hails progress in Beijing
Trump warns Britain on China ties as Starmer hails progress in Beijing
 Israel releases 15 Palestinian bodies as truce deal shifts to next phase
Israel releases 15 Palestinian bodies as truce deal shifts to next phase
 WHO sees low risk of Nipah virus spreading beyond India
WHO sees low risk of Nipah virus spreading beyond India
 Trump, Democrats say deal reached to avert shutdown
Trump, Democrats say deal reached to avert shutdown






