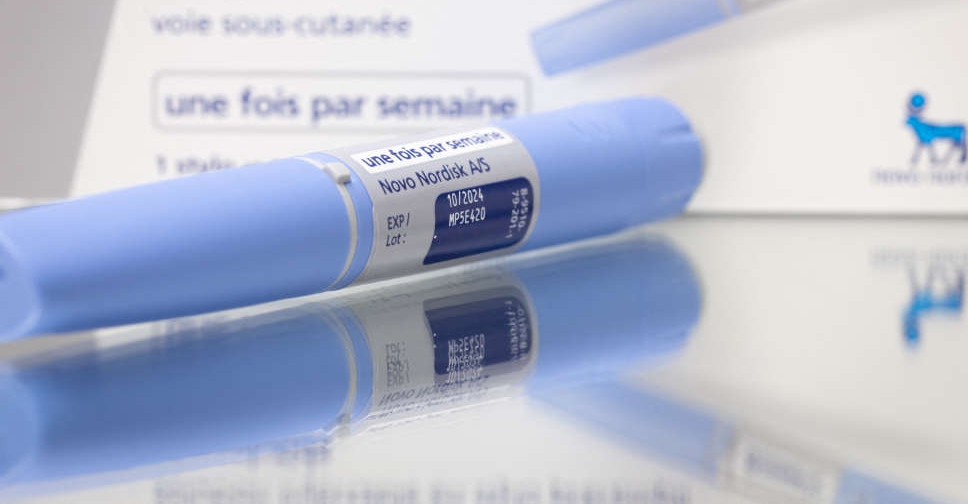
Several people were taken to hospital in Austria after using suspected counterfeits of the diabetes drug Ozempic, according to the federal health safety office.
The patients were reported to have suffered hypoglycaemia and seizures, serious side effects that indicate the product contained insulin instead of Ozempic's active ingredient, semaglutide, the BASG said in a warning issued on Monday.
Europe's medicines regulator last week warned about pre-filled pens falsely labelled as the diabetes drug, and Novo Nordisk has flagged a surge in counterfeit versions of Ozempic as well as its weight-loss drug Wegovy online.
Though only Wegovy is approved for obesity, the fact that Ozempic also leads to dramatic weight loss has led people in the US and Europe to use the drug "off-label", meaning not for its approved use.
This has led to a shortage of Ozempic, which has been exploited by criminal organisations, the BASG said.
The BASG did not give an exact number of patients affected and how severely. A spokesperson was not available on Tuesday.
Austria's criminal intelligence service said on Monday that the affected batch probably came from a source other than a pharmacy and stocks of it may still be in circulation.
While the currently suspected counterfeits are packs of 1-milligram strength, it cannot be ruled out that pre-filled pen packs with different strengths are also affected, the service said.




 Explosion at mosque in Pakistan's capital kills 31
Explosion at mosque in Pakistan's capital kills 31
 Hong Kong media tycoon Jimmy Lai to be sentenced on February 9
Hong Kong media tycoon Jimmy Lai to be sentenced on February 9
 More storms coming as Leonardo swells rivers in Spain and Portugal
More storms coming as Leonardo swells rivers in Spain and Portugal
 Danone recalls more infant formula in Europe
Danone recalls more infant formula in Europe
 UN chief calls New START treaty expiration 'grave moment'
UN chief calls New START treaty expiration 'grave moment'







