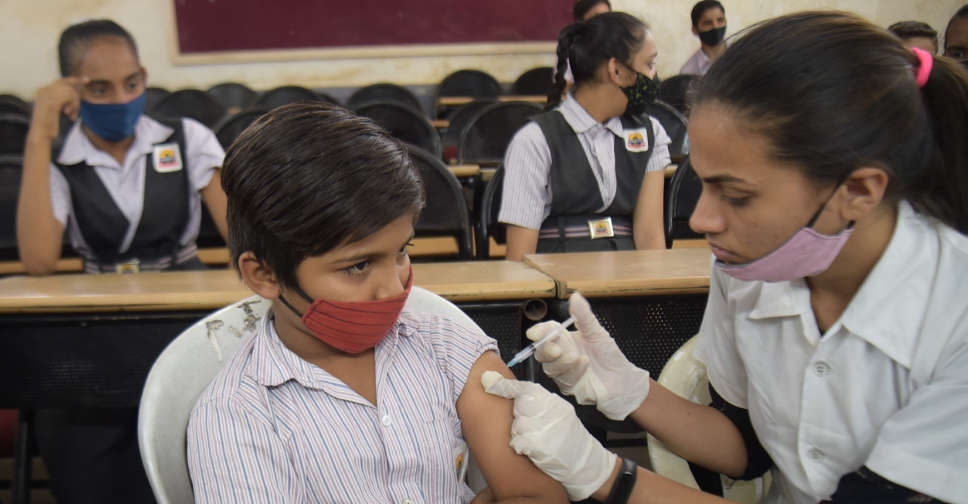
Novavax Inc said on Tuesday its COVID-19 vaccine has got emergency-use authorization from the Drugs Controller General of India for children aged 12 to 17 years.
The authorisation is a global first for the age group for the vaccine, which is manufactured and marketed in India by the Serum Institute of India under the brand name Covovax.
Novavax last month said its vaccine was 80 per cent effective against COVID-19 in a late-stage trial testing the shot in 2,247 teens aged 12 to 17 years. Read full story
The company said on Tuesday its vaccine produced an immune response in the same age group in a mid- to late-stage study involving 460 Indian adolescents.
Covovax is the fourth COVID-19 vaccine to be authorised for adolescents aged 12 years and older in India after Corbevax, ZyCoV-D and Covaxin.
India, which had so far been vaccinating children aged 15 and above, started administering doses of Corbevax last week to children aged 12 to 14.
The country's drug regulator in December authorised Novavax's COVID-19 vaccine for people aged 18 years and above.




 Trump warns Britain on China ties as Starmer hails progress in Beijing
Trump warns Britain on China ties as Starmer hails progress in Beijing
 Israel releases 15 Palestinian bodies as truce deal shifts to next phase
Israel releases 15 Palestinian bodies as truce deal shifts to next phase
 WHO sees low risk of Nipah virus spreading beyond India
WHO sees low risk of Nipah virus spreading beyond India
 Trump, Democrats say deal reached to avert shutdown
Trump, Democrats say deal reached to avert shutdown
 Vietnam police seize tonnes of fake coffee products made from soybeans
Vietnam police seize tonnes of fake coffee products made from soybeans







