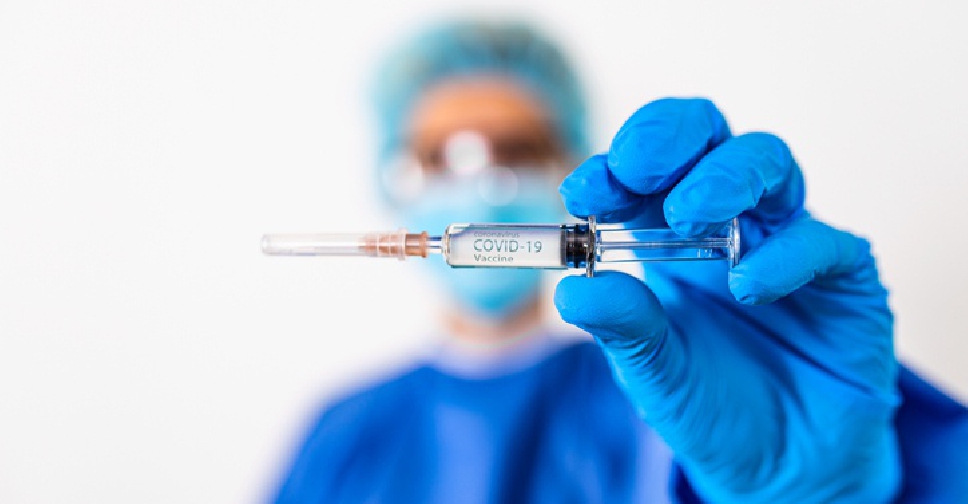
India's drug regulator on Tuesday allowed vaccine maker Serum Institute to enrol children aged 7-11 years for its COVID-19 vaccine trial as the country prepares to protect kids from the coronavirus.
It has already administered more than 870 million doses to adults among its population of nearly 1.4 billion.
"After detailed deliberation, the committee recommended for allowing enrolment of subjects of 7 to 11 years of age group as per the protocol," a subject expert panel of the Central Drugs Standard Control Organisation said.
Serum Institute is already conducting a trial of its COVID-19 vaccine, a domestically produced version of US drugmaker Novavax's shot, in the 12-17 age group and has presented safety data for an initial 100 participants.
So far, only drugmaker Zydus Cadila's DNA COVID-19 vaccine has received emergency use approval in India to be used in adults and children aged 12 years and above.




 UK, France, others state Gaza humanitarian deterioration of serious concern
UK, France, others state Gaza humanitarian deterioration of serious concern
 China encircles Taiwan in massive military display
China encircles Taiwan in massive military display
 Kremlin says Russia is toughening its stance on Ukraine after drone attack
Kremlin says Russia is toughening its stance on Ukraine after drone attack
 Turkey detains 357 ISIS suspects nationwide after deadly clash
Turkey detains 357 ISIS suspects nationwide after deadly clash
 Israel defends Somaliland move at UN amid concerns over Gaza motives
Israel defends Somaliland move at UN amid concerns over Gaza motives







